Navigating the Gauntlet: A Guide to the FDA's Evolving Cybersecurity Guidance for Medical Devices
The digital transformation of healthcare has ushered in an era of unprecedented innovation, with connected medical devices at the forefront. From...
3 min read
![]() Palindrome Technologies
:
Jun 9, 2025 5:08:05 PM
Palindrome Technologies
:
Jun 9, 2025 5:08:05 PM
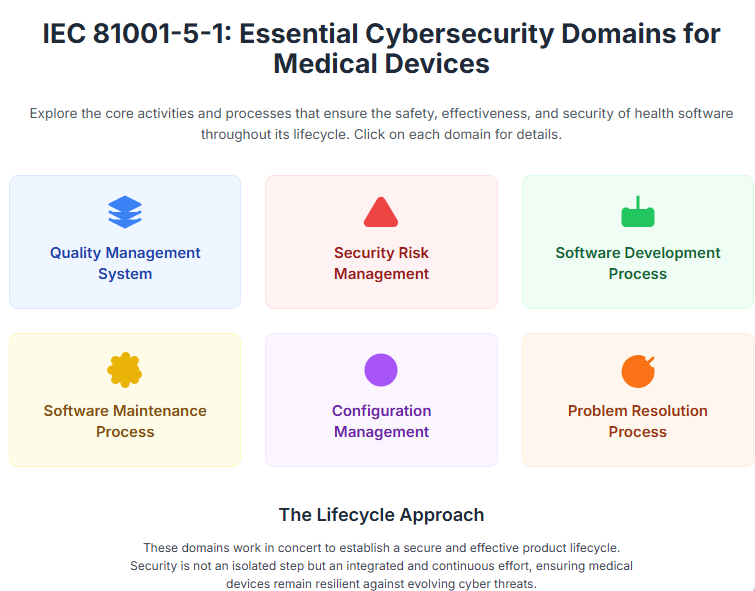
In today's interconnected healthcare landscape, the cybersecurity of medical devices is paramount. As medical technology becomes more sophisticated and integrated, so too do the potential threats and vulnerabilities. For original equipment manufacturers (OEMs), navigating this complex environment while ensuring patient safety and meeting regulatory requirements presents a significant challenge.
Fortunately, industry standards such as IEC 81001-5-1:2021, "Health software and health IT systems safety, effectiveness and security - Part 5-1: Security - Activities in the product life cycle" provide a robust framework to decisively address these critical concerns.
Adopting and rigorously implementing IEC 81001-5-1 offers a multitude of strategic advantages for medical device manufacturers:
The U.S. Food and Drug Administration (FDA) has, with increasing stringency, underscored the criticality of cybersecurity for medical devices. The compelling advantage of implementing IEC 81001-5-1 is its inherent and direct alignment with the FDA's rigorous expectations. The standard's core tenets, secure product development lifecycle, comprehensive risk management, proactive vulnerability identification and remediation, and meticulous documentation, directly reinforce the principles articulated in FDA cybersecurity guidance. Indeed, IEC 62443, a foundational series of standards closely related to IEC 81001-5-1, enjoys explicit recognition by the FDA. By adhering to the directives of IEC 81001-5-1, medical device OEMs are not merely engineering more secure products; they are demonstrably committing to regulatory excellence, thereby potentially expediting market access and fortifying their standing within the industry.

For OEMs committed to implementing IEC 81001-5-1 and achieving an unparalleled medical device cybersecurity posture, Palindrome Technologies stands as the definitive and optimal partner. Our distinguished expertise is uniquely positioned to deliver superior results:
By forging a partnership with Palindrome Technologies, medical device OEMs can confidently navigate the formidable complexities of cybersecurity, decisively reduce threats and vulnerabilities, satisfy stringent regulatory mandates, and ultimately deliver safer, more effective, and more trusted products to healthcare providers and patients worldwide. Elevate your product security; it is an unequivocal imperative for market leadership and patient well-being.
The digital transformation of healthcare has ushered in an era of unprecedented innovation, with connected medical devices at the forefront. From...
The landscape of healthcare data privacy is undergoing a significant transformation in 2025, with federal regulators rolling out some of the most...
If you sit in a compliance seat, you already know the uncomfortable truth: HIPAA is no longer being enforced like a “policy and training” regime. HHS...
The Health Insurance Portability and Accountability Act (HIPAA) is more than just a set of rules; it's the bedrock of patient trust in the digital...